To stop contamination in the sampling and testing process, the QC Department ought to adhere to demanding aseptic techniques.
Guarantee personnel entirely comprehend the proven acceptance requirements for microbial counts. Give examples and scenarios to improve comprehension and a chance to establish deviations.
The amount and types of micro-organisms that could create in many pharmaceutical dosage varieties is drastically influenced with the presence of substances with antimicrobial Homes. Antimicrobial active substances can be divided into 3 groups, as follows:
If deviations from acceptance standards are recognized, sustain specific deviation reports. Incorporate information on the root cause Investigation, corrective actions taken, and preventive measures implemented to stay away from long term occurrences.
This cookie is ready by Spotler and suppliers the UTM values with the session. UTM values are precise text strings which might be appended to URLs that enable Communigator to trace the URLs and also the UTM values whenever they get clicked on.
Set up obvious interaction protocols within the Firm regarding deviations. Make sure appropriate departments are informed promptly and that there is a specified chain of communication for managing deviations.
During the celebration of deviations or non-conformities identified for the duration of microbial limit testing, the QA Office will take the guide in conducting comprehensive investigations.
Disclaimer: You at the moment are leaving PharmiWeb.com Web site and are likely to a website that isn't operated by us. We are not chargeable for the content or here availability of linked internet sites.
We make no illustration or warranty regarding the precision of the information contained in the joined web sites. We advise that You usually verify the data attained from joined Web sites right before acting on this info.
Among the first responsibilities from the QC Office is to determine and put into practice robust sampling protocols. This consists of deciding the appropriate sampling points, frequencies, and volumes to obtain agent samples of raw materials and completed products. Enough sampling is important for precise microbial limit testing.
From a pure microbiological viewpoint putting on an overall doesn’t make sense aside from the marketing of the Mindset of Functioning cleanly and neatly. By now just after 1–two h the overall bears just as much contamination as the personal clothes. Instructions for outfits are nevertheless also necessary to market occupational security and overall health (see Sect.
Temperature has a robust influence on regardless of whether an organism can survive or thrive. Temperature exerts its impact indirectly as a result of drinking water (that has to become inside the liquid point out), and instantly through its affect on the organic and natural molecules composing the residing cells.
The procedure of bioburden testing for producing (Uncooked resources and packaging procedures) in or on a professional medical product has the next 4 unique stages: Microorganism Restoration, website Enumeration of microorganisms, Bioburden characterization, Validating the method.
Concurrently, the harmful metabolites of microorganisms plus some pathogenic microorganisms can also cause adverse reactions or secondary bacterial infections to clients. For that reason, microbial limit testing for non-sterile drug products has become the critical measures to make sure the standard, safety and usefulness of medication.
 Ariana Richards Then & Now!
Ariana Richards Then & Now!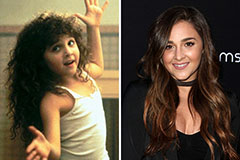 Alisan Porter Then & Now!
Alisan Porter Then & Now! Jurnee Smollett Then & Now!
Jurnee Smollett Then & Now! Marla Sokoloff Then & Now!
Marla Sokoloff Then & Now! Mike Smith Then & Now!
Mike Smith Then & Now!